
By Colin Jeffrey,
Empa researchers have demonstrated the use of pyrite (fool’s gold) as energy production moves towards solar and wind-powered alternatives, battery systems to store intermittently-produced electricity have never been more important. Unfortunately, many of the materials needed to make high-performance batteries for this purpose are rapidly diminishing and becoming increasingly expensive as a result. Now researchers from Empa and ETH Zurich have created a new type of storage battery that is made from a range of cheap and abundant materials and shows promise for high-efficiency performance.
The prototype battery uses nanocrystals composed of iron sulfide, better known as pyrite or “fool’s gold”, as the cathode, sodium as the electrolyte and magnesium for the anode. All of these ingredients are relatively inexpensive and abundant, with materials such as iron sulfide nanocrystals being made by simply milling sulfur with dry metallic iron, whilst a kilogram of magnesium is some 15 times cheaper than a comparable quantity of lithium. In addition, iron, magnesium, sodium, and sulfur are 4th, 6th, 7th, and 15th in order of abundance on Earth by mass, respectively.

Electronmicroscope image of Pyrite nanocrystals(Credit: Empa)
Further savings could also be realized in the construction of the battery, as aluminum foil is perfectly adequate to accumulate and conduct electricity from it, where as lithium ion (Li-ion) batteries need comparatively expensive copper foil to perform the same role.
In use, when the battery discharges, sodium ions suspended in the electrolyte move towards the cathode, where they accumulate. When the battery is recharged, the pyrite re-releases the sodium ions back to the electrolyte. Verification of the operation of this sodium-magnesium hybrid storage cell has already been demonstrated in the laboratory, with the test battery already having achieved 40 charging and discharging cycles with no change to its performance.
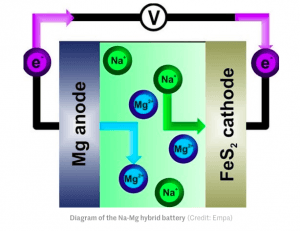
Diagram of the Na-Mg hybrid battery(Credit: Empa)
Though the output of the new battery is presently lower than that of a comparable size Li-ion, the researchers believe that the inexpensive and scalable nature of the new device is such that it could be used to construct enormous storage cells for power stations. One such suggestion from the scientists is that a sufficiently large battery might be used to temporarily store an entire year’s output from a nuclear power station, for instance. In essence, if a big enough battery is constructed, it could potentially store many terawatt-hours of energy.
“The battery’s full potential has not been exhausted yet,” said Prof. Dr. Maksym Kovalenko, who teaches at ETH Zurich’s Department of Chemistry and Applied Biosciences as well as researching at Empa. “If we refine the electrolytes, we’re bound to be able to increase the electric voltage of the sodium-magnesium hybrid cell even further and to extend its cycling life.”
Investors are being sought to support further research and bring the technology to market.”
